- Chinese website
- Sitemap
- Mobile site

- Customer service hotline:400-666-5548

Product Description
Product Name: protective clothing suit for medical use non sterile type
Applicable People:Adult
Standard:GB19082-2009
Material: composite non-woven fabric material
Place of Origin:Hunan, China
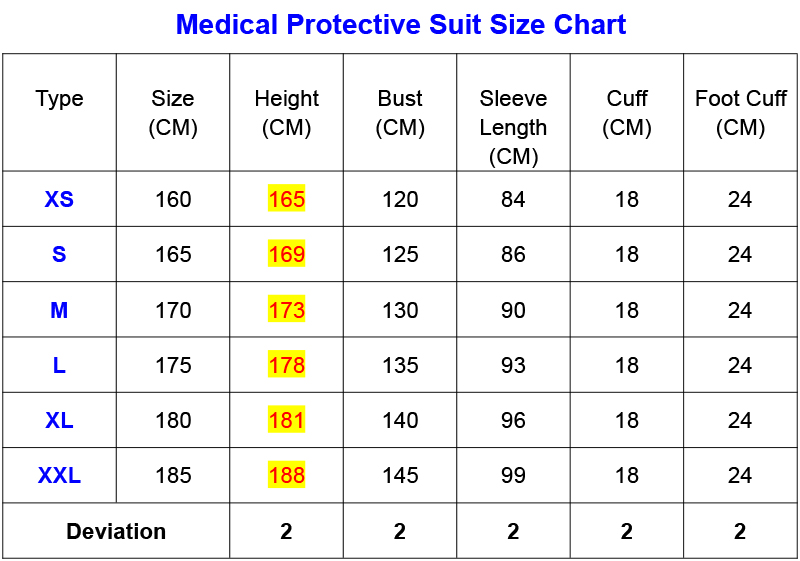
Size:A/XXL, A / XL, A / L, A / M, A / S, A / XS.
Validity Period:2 Years.
Packing:1pcs/Pack
Scope of Application:To provide barrier and protection for clinical medical personnel in their work when contacting with patients with potential infectious blood, body fluids, and secretions .The product is for one-time use only. It should not be used for isolation of critical care areas (rooms ) and other places with strict control of microbial indicators .
Warnings
1.This product is for one-time use. It can not be used if the package is damaged.
2. Repeated use is not allowed. Destroy it after use
Storage Condition
The package should remain intact, kept away from humid and high temperature, and stored in ventilative place with no corrosive gases.
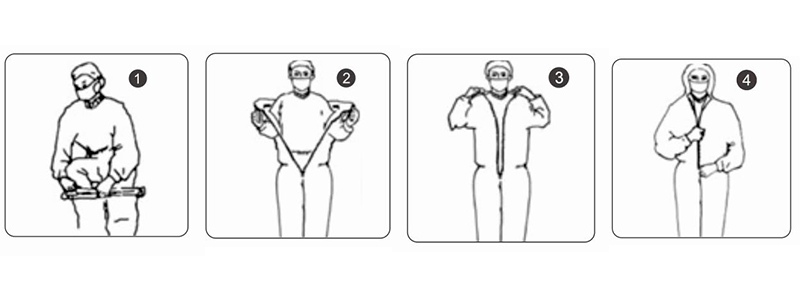
Direction for Use
1.Take the suit out of the sealed bag.
2.Put on the suit from bottom to top
3.Pull the zipper to a suitable position. Remove the sealing tape and stick it on clothing then zip up

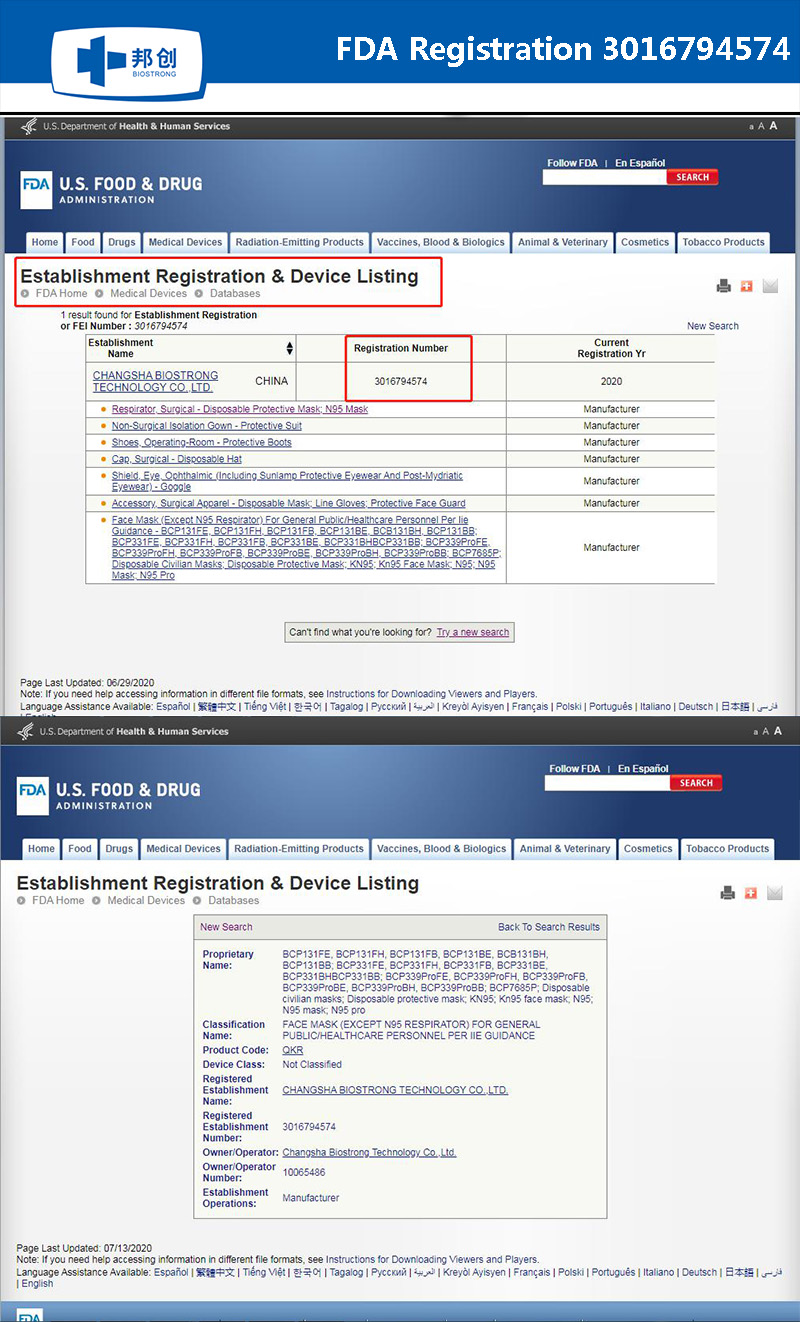
The manufacturer(MFT) of Pandemic prevention products-Changsha Biotrong Biotechnology Co., Ltd. all rights reserved湘ICP备18002869号-1Technical support:Hnjing
 WeChat
WeChat